SEM Scanner recognized for role in transforming pressure ulcer care to save money and release productivity for a public health issue costing the NHS over £2B per annum
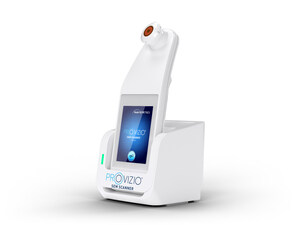
MANCHESTER, England, Aug. 3, 2017 /PRNewswire/ -- At this year's Patient Safety Awards, Bruin Biometrics and Isle of Wight NHS Trust were awarded Best Product or Innovation for Patient Safety for use of the SEM Scanner, a non-invasive device for early detection of pressure ulcers ("bedsores"), by the prestigious Health Service Journal. An estimated 18 to 25 percent of patients in acute care and long-term care settings suffer from pressure ulcers, which cost the NHS £2.1bn per annum.
The winning project evaluated how early detection can support nurses in delivering effective care, has resulted in a complete elimination of hospital-acquired pressure ulcers in those wards. The judges felt that this was truly transformational for the sector, with a visible and measurable impact on patient safety improvement.
"We are extraordinarily proud of the tissue viability and nutrition team and what they have been able to accomplish. We have since expanded the program, and have not seen a single pressure ulcer in the past two months on patients that we have scanned where we implemented the SEM Scanner," said Glenn Smith, Tissue Viability Nurse and Patient Safety Lead at St. Mary's Hospital NHS Isle of Wight NHS Trust, and Finalist for British Journal of Nursing's Pressure Care Nurse of the Year award.
"The information from the scanner allows us to intervene before the point that pressure injuries become obvious. We are starting to scan people on admission, and we also now can tell within twenty-four hours if a specific intervention is working and can personalize patient care plans appropriately. Now, where we have the scanners, I don't have to wait until the skin goes red to avoid a pressure ulcer," he continued.
This year saw fierce competition for these coveted awards with over 550 submissions, of which only 170 made it to the final cut, and from those 21 deserving winners were decided by our expert panel of judges.
"The Patient Safety Awards, now in its 9th year, is an annual celebration of the NHS's fantastic efforts to improve the quality of healthcare. Our independent judges, drawn from across the health service, have chosen the very best of the best – a collection of talent that every year helps the NHS continue to be a global leader in safety," said Shaun Lintern, Senior Patient Safety Correspondent. "This is one of the toughest times for the NHS as patient demand and acuity continue rising and there are workforce shortages across all health sectors. In that context the success of each of the winners and shortlisted projects are that much more deserving of our thanks and recognition."
This is the eighth award for Bruin Biometrics and its partners, including awards from British Journal of Nursing, Journal of Woundcare, and Frost & Sullivan.
"We are thrilled to be recognized for our innovation and commitment to supporting nurses achieve significant and sustainable reductions in pressure ulcer occurrence to save lives, cost and time," said Colin Priestley, EMEA Director for BBI. "The clinicians we work with are tirelessly committed to their professions and their patients. It is a true pleasure to see them be rewarded with results for embracing new technology in their practice."
About Pressure Ulcers
Pressure ulcers (also known as "bedsores") can lead to pain, disfigurement, infection and deadly complications that kill more people annually than any form of cancer except lung cancer. The National Health Service spends £2.1bn – four percent of its annual budget – on bedsore treatment and prevention. The ulcers result from pressure involving shear and/or friction and sustained tissue distortion causing localized damage to the skin and underlying tissue, usually around areas of bony prominence -- such as the vertebra, tailbone, heels, and hips.
Across Europe and the United States, an estimated 18 to 25 percent of patients in both acute care and long-term care settings suffer from pressure ulcers, which disproportionately impact the elderly and patients with limited mobility. There are some 2.5 million pressure ulcer cases annually in the European Union, and nearly 500,000 in the United Kingdom.
Some 2.5 million Americans develop pressure ulcers annually in acute care facilities, and 60,000 Americans die annually from pressure ulcer complications such as cancer, sepsis, cellulitis, and MRSA.
About BBI
Bruin Biometrics ("BBI LLC"), a pioneer in biometric-sensor based medical devices, develops point-of-care diagnostic solutions for early detection and monitoring of chronic, preventable conditions. The company's first product is the SEM Scanner, a hand-held non-invasive device that assesses sub-epidermal moisture, an early indicator for pressure damage (pressure ulceration, deep tissue injury). Pressure ulcers affect approximately 25 percent of acute care hospital and long-term care patients – typically the elderly and immobile. SEM Scanner is CE Mark approved. It is currently in full commercial launch in the United Kingdom, Ireland and Canada. SEM Scanner is pending approval from FDA, and is not available for sale in the United Sales.
BBI is also developing OrthoSonos, a non-invasive device for real-time orthopedic joint monitoring and assessment of prosthetic implant failure; and P02M, the first device for monitoring tissue oxygenation at a specific location in real time. P02M is initially being tested for continual monitoring of tissue and vascular viability in the feet of diabetics. Diabetes can cause peripheral artery disease and peripheral neuropathy, putting patients at risk for foot ulcers.
BBI is based in Los Angeles, USA and maintains a European office in Manchester, UK.
For additional information, visit www.bruinbiometrics.com. Follow BBI on Twitter at https://twitter.com/bruinbiometrics.
Logo - https://mma.prnewswire.com/media/3604/bruin_biometrics__llc_logo_3674_21079_.jpg





Share this article