The publication titled "An optimized IL-12-Fc expands its therapeutic window, achieving strong activity against mouse tumors at tolerable drug doses" was published on April 13th in Cell Press's Med.
WALTHAM, Mass., April 17, 2023 /PRNewswire/ -- Dragonfly Therapeutics, Inc., a clinical stage biotechnology company developing novel immunotherapies, today announced the April 13th publication of preclinical data in Cell Press's Med supporting DF6002 as a promising treatment option for cancer patients. DF6002 is Dragonfly's novel half-life extended interleukin-12 (IL-12) cytokine immunotherapy, currently in Phase 1 clinical development with dose escalation progressing successfully in patients as a monotherapy and in combination with nivolumab, in the U.S. and in Europe.
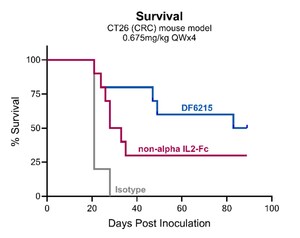
Systemic IL-12 cytokine therapy was historically associated with severe toxicities due to its narrow therapeutic index. Dragonfly's preclinical data demonstrates that DF6002 is well tolerated when administered systemically and results in potent anti-tumor responses in multiple preclinical models as monotherapy and in combination with checkpoint inhibitors. Dragonfly's novel DF6002 IL-12 is designed for half-life extension which alters IL-12's pharmacodynamic response profile and expands its therapeutic index. "We are excited about these findings, which provide compelling evidence supporting our ongoing Phase I clinical trial, which is evaluating the safety and tolerability of DF6002 in patients with advanced solid tumors," said Joseph Eid, MD, Dragonfly's President of Research and Development. "Given the encouraging profile we have seen both in preclinical models and in the clinic to date, we are accelerating DF6002's development across a range of indications and combinations."
About DF6002
DF6002, Dragonfly's extended half-life IL-12 cytokine, is an investigational immunotherapy being evaluated alone and in combination with nivolumab in participants with locally advanced or metastatic solid tumors (NCT04423029). DF6002 is a monovalent IL-12 immunoglobulin Fc fusion protein proposed to achieve strong anti-tumor efficacy by establishing an inflammatory tumor microenvironment necessary for productive anti-tumor responses. DF6002 has the potential to stimulate effective anti-tumor immunity in patients who are not eligible or not adequately responding to current therapies. DF6002 is the most advanced in a pipeline of cytokines that Dragonfly is developing to address the high unmet need in patients with advanced cancer.
About Dragonfly
Dragonfly Therapeutics is a clinical-stage biopharmaceutical company committed to discovering, developing, and commercializing therapies that use its novel bispecific antibody technology to harness the body's immune system to bring breakthrough treatments to patients. In addition to its wholly-owned clinical assets and multiple assets in the clinic with partners, Dragonfly has a deep pipeline of wholly-owned preclinical candidates developed using its proprietary platforms as well as productive collaborations with Merck, AbbVie, Gilead and Bristol Myers Squibb in a broad range of disease areas.
For more information visit:
www.dragonflytx.com
https://www.linkedin.com/company/dragonfly-therapeutics-inc./
https://twitter.com/dragonflytx
DRAGONFLY MEDIA CONTACT:
Anne Deconinck | anne@dragonflytx.com
Logo - https://mma.prnewswire.com/media/390962/Dragonfly_Therapeutics_Inc_Logo.jpg






Share this article