FDA Purchases XPS license - Developed by RCPE and Distributed by InSilicoTrials - as In-House Simulation Tool
MILAN, April 4, 2024 /PRNewswire/ -- The Research Center Pharmaceutical Engineering (RCPE) and InSilicoTrials are pleased to announce a significant milestone in their collaborative journey with the United States Food and Drug Administration (FDA). The FDA has recently purchased an XPS (eXtended Particle Simulations) license as an in-house simulation tool, reflecting a trend in the agency's engagement with advanced computational technologies.
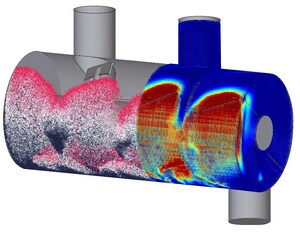
XPS is a state-of-the-art Discrete Element Method (DEM) simulation software, developed by RCPE and distributed globally by InSilicoTrials through an exclusive partnership agreement. It is renowned for its ability to predict granular processes in great detail, enhancing the understanding, prediction and control of pharmaceutical unit operations, thus leading to improved efficiency and product quality.
XPS relies on advanced contact models to accurately describe the flow behavior of granular materials. Optimized for speed with algorithms designed for modern Graphics Processing Units (GPUs), XPS can handle simulations with up to 100 million particles, ensuring rapid execution and enabling process optimization on even standard desktop machines.
The software's adoption followed a successful project between the FDA and RCPE, which focused on studying powder flow and mixing in a continuous manufacturing line using high-fidelity DEM simulations. The collaboration u-sed XPS to predict Residence Time Distributions (RTDs), for the development of a flowsheet modeling framework based on reduced-order models. During this process, the predicted RTDs were successfully validated with experimental data collected at the state-of-the-art continuous manufacturing facility at RCPE's pilot plant in Graz, Austria. This framework was used to design an easily configurable control strategy evaluation tool for continuous direct compression processes, allowing for the evaluation of various control strategies.
The DEM simulations conducted using XPS showcased the software's capability in delivering results at record speeds. RCPE and InSilicoTrials view the success of these simulations and the FDA's subsequent adoption of XPS as a highlight of the software's potential to support the pharmaceutical industry's transition to more advanced manufacturing processes. The results from this collaboration have been shared at numerous conferences and publications in peer-reviewed scientific journals will follow.
Highlights for pharma: Leveraging cutting-edge algorithms, XPS helps to optimize R&D processes by reducing the need for costly and time-consuming physical experiments. Designed specifically for pharma, XPS accurately predicts powder behavior using consumer-grade GPUs to accelerate simulations, while its multi-physics features and digital twin capabilities streamline pharmaceutical manufacturing, enhancing both product development and operational efficiency.
About RCPE: The Research Center Pharmaceutical Engineering (RCPE) is a global leader in pharmaceutical process engineering. We enable our partners to develop and manufacture innovative medicines. Through our science, we help to realize tomorrow's therapies and improve patients' lives worldwide. RCPE is funded within the framework of COMET - Competence Centers for Excellent Technologies by BMK, BMAW, Land Steiermark, and SFG. The COMET program is managed by the FFG.
About InSilicoTrials: InSilicoTrials, founded by experts in life sciences, cybersecurity, and digital innovation, aims to transform healthcare with a cloud simulation platform that leverages AI and in silico techniques to predict the safety and efficacy of compounds. This approach assists Pharma, Biotech companies, and researchers in advancing R&D more efficiently and cost-effectively by minimizing the need for extensive non-clinical testing and clinical trials.
Photo - https://mma.prnewswire.com/media/2379427/InSilicoTrials.jpg





Share this article