First Successful Clinical Implantation of Transcatheter Pulmonary Valve VenusP-Valve was Successfully Completed in Brazil
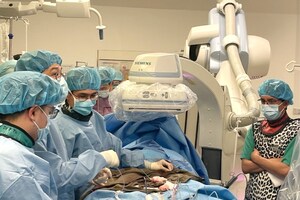
SAO PAULO, Dec. 22, 2017 /PRNewswire/ -- On December 19, 2017, local time, 4 clinical implantations of transcatheter pulmonary valve VenusP-Valve were successfully completed in Brazil by Professor Carlos Pedra from Dante Pazzanese Institute of Cardiovascular Technology in Sao Paulo, Brazil.
After Chile and Argentina, Brazil becomes the third Latin American country to apply the valve of Venus Medtech (Hangzhou) Inc. It also marks the first application of China-made heart valves in Brazilian history.
At present, in every 10,000 newborns in the world, there are 16 newborns that will need surgery because of congenital heart defects. This kind of surgery can cause mass pulmonary regurgitation. Currently the interventional pulmonary valve products in the global market can only treat patients with special anatomical structure and light illness. As a result, a large number of patients with complex anatomical structure and poor right heart function are in urgent need of an interventional pulmonary valve product that could treat different anatomical structure and be operated with ease, safety and stability.
Venus Medtech (Hangzhou) Inc. has developed and manufactured transcatheter pulmonary valve VenusP-Valve, the world's first self-expanding interventional pulmonary valve. With unique double trumpet-shaped designs and laser cutting technology for valve stent, VenusP-Valve is endowed with a strong radial support force, stable anchoring, and easy release. No movement or displacement will occur during the release process. It can meet the needs of pulmonary arteries with different anatomical structures and features the only interventional pulmonary valve in the world that can treat patients with a large size of right ventricular outflow tract.
After the first successful human implantation by Academician Ge Junbo in Zhongshan Hospital, Shanghai on May 25, 2013, VenusP-Valve has entered more than 20 countries in Asia, Europe and America. At present, the clinical trial of the Chinese Food and Drug Administration (CFDA) has been finished; the process of European CE clinical trial has entered more than half of the period; communication with the United States' Food and Drug Administration (FDA) has been completed, with the start of the clinical trial before launching in USA. It is hoped that VenusP-Valve, with superior clinical results, can be approved as early as possible to provide optimal treatment for more patients.
Photo - http://mma.prnewswire.com/media/622562/Venus_Medtech.jpg



Share this article