GemVax & KAEL reports further positive data from GV1001 Phase II Alzheimer's Disease trial
SEOUL, Nov. 23, 2020 /PRNewswire/ -- GemVax & KAEL Co., Ltd. a clinical stage company developing a novel peptide drug based on telomerase modification has released further positive data at the Korean Dementia Association's Fall Conference from a Phase II trial in Alzheimer's disease of lead candidate GV1001. Previously the company announced GV1001 was well tolerated and safe and the primary endpoint was achieved with notable improvements in Severe Impairment Battery (SIB) scores. The newly released data also shows a statistically significant improvement in the Neuropsychiatric Inventory (NPI) and an improvement trend in Alzheimer's Disease Cooperative Study-Activities of Daily living (ADCS-ADL). Based on these results, the company is now planning to submit a Phase III IND application.
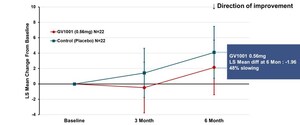
GemVax's GV1001 Phase II clinical trial evaluated the safety and efficacy of subcutaneous injection of GV1001 0.56 mg or 1.12 mg for six months in patients with moderate-to-severe Alzheimer's disease already receiving donepezil for more than three months, at 12 medical institutions. PI Professor Seong-Ho Koh, at Hanyang University Guri Hospital said, "The Severe Impairment Battery (SIB), the primary endpoint of this clinical study, showed an statistically significant greater improvement in the group treated with GV1001 1.12 mg compared with the control group treated with donepezil alone, which showed 7.11 difference in the overall SIB score. Among the secondary outcome results, the group treated with GV1001 1,12mg had a significant greater improvement in the Neuropsychiatry Inventory (NPI) scores compared to the control group.
Furthermore, although found to be statistically not significant, the other secondary outcome, the Alzheimer's disease cooperative study, Activities of Daily living (ADCS-ADL) score showed an apparent trend in improvement similar to the SIB results."
Kim Sang-Jae, chairman of GemVax "Combined with the notable improvement in SIB scores in patients with moderate-to-severe Alzheimer's disease, these significant results in NPI and ADCS-ADL scores are extremely encouraging. We have decided to submit a Phase III in Korea to see if it is possible to demonstrate improvement in a larger group of moderate-to-severe patients."
Professor Koh, agrees, "If efficacy and safety results of GV1001 in the phase III clinical trial are the same as or superior to the Phase II clinical trial, it will be able to establish itself as a game-changer in Alzheimer's disease treatment".
For more information visit www.gemvax.com
Media Contact
Richard Hayhurst
T: +44 7711 821 527
E: Richard@rhapr.eu






Share this article