InSilicoTrials' research paper published in the Special Issue "Modeling for Advancing Regulatory Science" by Annals of Biomedical Engineering (ABME)
MILAN, Jan. 26, 2023 /PRNewswire/ -- InSilicoTrials, leading player in using artificial intelligence (AI) and Modeling & Simulation (M&S) for drug and medical device development, is proud to announce that its research paper, Accelerating Digitalization in Healthcare with the InSilicoTrials Cloud-Based Platform: Four Use Cases, has been selected to be part of the Special Issue "Modeling for Advancing Regulatory Science", released by Annals of Biomedical Engineering (ABME) in occasion of the 50th anniversary of the Biomedical Engineering Society.
Computational modeling and simulation can provide unique insights on how human biology and physiology work and why. In November 2022, the FDA published its first report on the Successes and Opportunities in Modeling & Simulation for FDA, highlighting a selection of modeling and simulation case studies from across FDA and illustrating how these technologies have already been embraced and are playing an important role in helping FDA promote public health.
The Special Issue, which was published on December 23rd, 2022, features an editorial by Tina M. Morrison, Joel D. Stitzel & Steve M. Levine - FDA's office of Regulatory Science and Innovation – entitled Modeling and Simulation in Biomedical Engineering: Regulatory Science and Innovation for Advancing Public Health. The authors highlight how the potential of CM&S (Computer Modeling and Simulation) to overcome obstacles in enhancing medical treatments has been well-established.
The editorial recalls that in the last few of years, Congress has urged FDA "to engage with device and drug sponsors to explore greater use, where appropriate, of in silico [clinical] trials for advancing new devices and drug therapy applications." This highlights a "shift toward a greater reliance on digital evidence from in silico methods in healthcare, opening the door to regulatory pathways in which a collection of computer-based models may be used to reduce, refine, and partially replace both in vivo animal and human experimentation for regulatory purposes."
The editorial concludes that "the practices we establish today will determine the health of all future generations. The time is now to make the necessary changes, not just to advance and translate science, but to create an equitable and foremost healthcare system based upon it."
InSilicoTrials is proud and honored to be part of this process, together with its strong network of scientific partners, creating the biggest collection of in silico tools, and democratizing access to this technology for the industry.
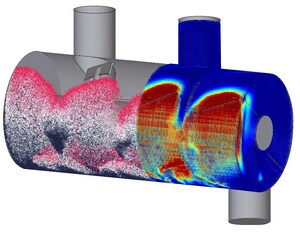
InSilicoTrials' paper discusses four use cases of in silico trials performed using the InSilicoTrials.com platform for modeling and simulation, providing insights on how the technology can be used to accelerate the development of new treatments and medical devices. The first application illustrates how in silico approaches can improve the early nonclinical assessment of drug-induced cardiotoxicity risks. The second use case is a virtual reproduction of a bench test for the safety assessment of transcatheter heart valve substitutes. The third and fourth use cases are examples of virtual patients' generation to evaluate treatment effects in multiple sclerosis and prostate cancer patients, respectively.
"We are honored to have our work recognized by the FDA and ABME," said Luca Emili, CEO of InSilicoTrials. "Modeling and simulation are becoming increasingly important in the drug and medical device development process, and we believe that our cloud-based platform can play a significant role in helping to accelerate the uptake of these technologies."
An important step in this direction has already been made with the FDA Modernization Act 2.0 (FDAMA 2.0), a bill aiming to modernize and improve the regulatory process for drugs, with the goal of getting safe and effective products to market more quickly. Animal testing on new medications will no longer be required for FDA approval, thanks to legislation signed by US President Joe Biden at the end of last month.
The bill represents a radical shift in the way drugs and treatments will be developed.
The InSilicoTrials' platform is designed to make it easy for researchers and companies to access the latest modeling and simulation tools and resources, without the need for expensive hardware or software. The platform is regulatory compliant and has been used by several leading pharmaceutical and medical device companies.
The Special Issue is available for free download: https://link.springer.com/journal/10439/volumes-and-issues/51-1, while the InSilicoTrials' paper can be accessed directly via the following link: https://link.springer.com/article/10.1007/s10439-022-03052-6
The PDF version of this press release can be found here: https://insilicotrials.com/wp-content/uploads/2023/01/Press_Release_January23.pdf
InSilicoTrials is a company that specializes in using artificial intelligence (AI) and simulations to improve drugs and medical device development. It uses machine learning techniques to analyze large amounts of data and make predictions about the safety and efficacy of potential drug compounds and medical devices.
Our goal is to help pharmaceutical, medtech companies and researchers develop new drugs more efficiently and at a lower cost by reducing the need for traditional, time-consuming, and expensive clinical trials. With its cloud-based platform offering advanced M&S tools to perform in silico trials analyses, InSilicoTrials supports companies to integrate AI and simulation technology into their drug development workflows.
InSilicoTrials is currently working on four projects funded by the European Commission: In Silico World, SimCardioTest, Brainteaser & Disc4All.
Photo - https://mma.prnewswire.com/media/1989839/InSilicoTrials.jpg






Share this article