Lightpoint Medical Receives 2.4M Euro Grant to Support Clinical Study Aiming to Cut Breast Cancer Re-Operation Rates
CHESHAM, England, November 26, 2015 /PRNewswire/ --
Lightpoint Medical, an innovative medical device company specializing in imaging technologies, announced today that it has received a 2.4M Euro grant from the European Commission as part of the Horizon 2020 European Union Framework Programme for Research and Innovation, enabling the company to carry out a large-scale clinical trial in breast cancer.
(Logo: http://photos.prnewswire.com/prnh/20151016/277917 )
The study aims to cut the rate of re-operations in breast cancer, potentially preventing many thousands of women globally from facing additional surgery, and providing significant cost savings to national healthcare systems.
Surgery remains the primary treatment for breast cancer, yet its failure rate is high. A quarter of women undergoing surgery for breast cancer will have to deal with the distress of being recalled for a repeat operation. Today surgery fails so often because surgeons today have to rely solely on sight and touch to differentiate cancerous from healthy tissue. The consequence of which is a further procedure to remove any remaining cancer.
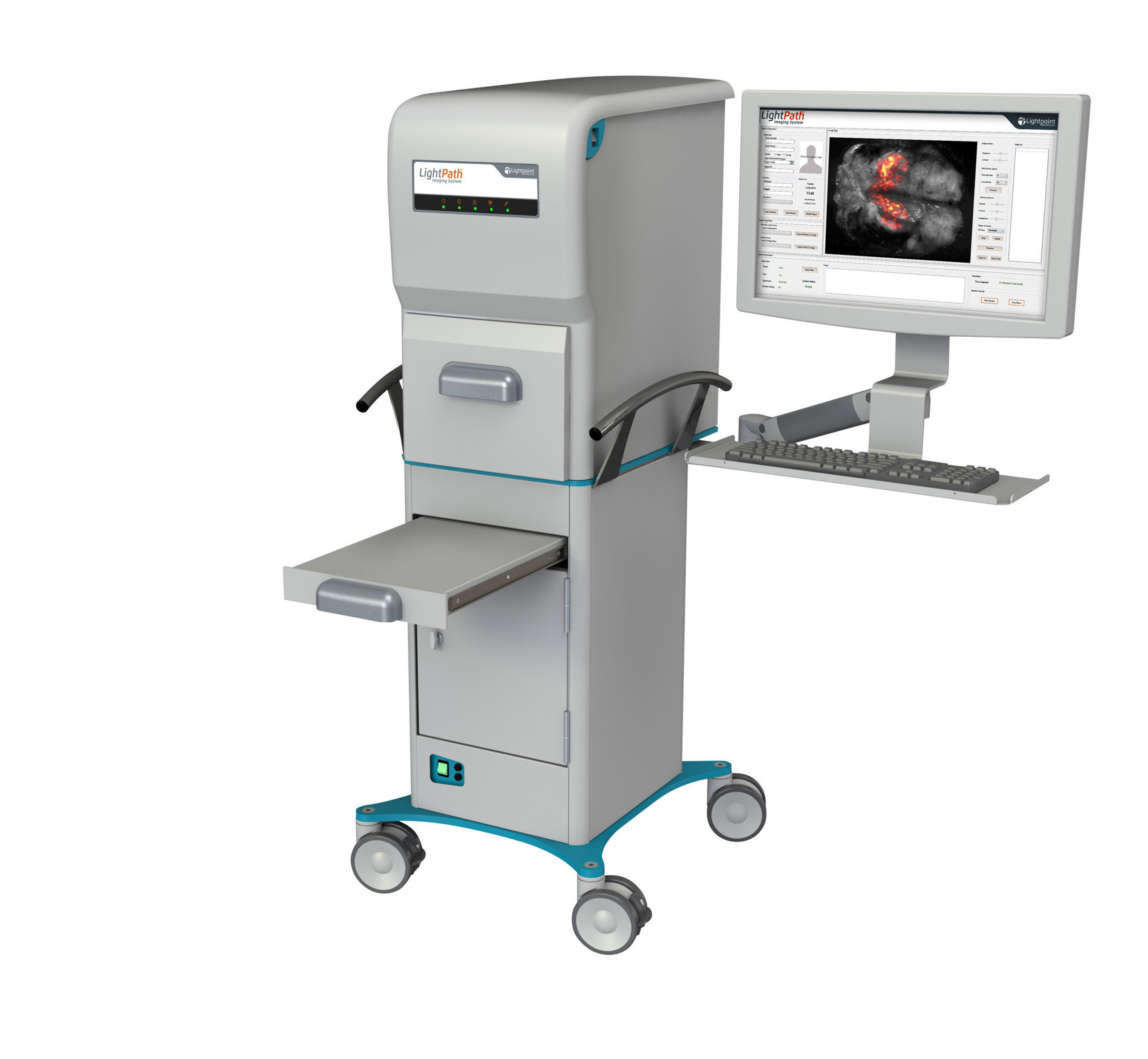
Using the Horizon 2020 funding, Lightpoint Medical will evaluate the LightPath™ Imaging System as a means of identifying cancer in a study involving over 300 breast cancer patients involving several hospitals in the UK, Germany, and France. The company recently announced the commercial launch of the LightPath™ system following CE Mark authorisation and pioneering feasibility studies in breast cancer surgery at Guy's & St Thomas' Hospital and prostate cancer at University College Hospital London.
As the first approved medical device for intra-operative molecular imaging in the world, LightPath™ accurately images numerous cancer types in real time and at a scale that can fit within an operating room. The LightPath™ Imaging System is designed to help surgeons ensure they have removed all cancerous tissue in a single operation. In addition to breast cancer surgery, the technology has potential application in a wide range of major cancer types, including prostate and lung cancer.
Speaking about the Horizon 2020 funding award, CEO of Lightpoint Medical, Dr David Tuch, said:
"We are deeply grateful to the Horizon 2020 program for supporting a large-scale international clinical trial of the LightPath™ technology. This award will help demonstrate the power of image-guided surgery to improve breast cancer surgery, and reduce the number of patients having to undergo repeat operations."
About Lightpoint Medical
Lightpoint Medical is an innovative medical device company dedicated to improving health outcomes for cancer patients through image-guided surgery. The company's ground-breaking molecular imaging technology has the potential to detect cancer in real-time during surgery and thereby reduce the need for repeat operations. For more information contact info@lightpointmedical.com or visit http://www.lightpointmedical.com
Horizon 2020 EU funding programme
Horizon 2020 is the biggest EU Research and Innovation programme ever with nearly €80 billion of funding available over 7 years (2014 to 2020) - in addition to the private investment that this money will attract. It promises more breakthroughs, discoveries and world-firsts by taking great ideas from the lab to the market. Small and Medium-sized Enterprises (SMEs) that are EU-based or established in a country associated to Horizon 2020 can now get EU funding and support for innovation projects that will help them grow and expand their activities into other countries - in Europe and beyond. The SME instrument addresses the financing needs of internationally oriented SMEs, in implementing high-risk and high-potential innovation ideas. It aims at supporting projects with a European dimension that lead to radical changes in how business (product, processes, services, marketing etc.) is done. It launches companies into new markets, promote growth, and create high returns of investment. The SME instrument addresses all types of innovative SMEs so as to be able to promote growth champions in all sectors.
This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 698263.
Share this article