Venus Medtech's Transcatheter Pulmonary Valve Debuts in Europe, Marking the Start of Global Clinical Trial
A milestone as a new generation of made-in-China medical products find their rightful place in European hospitals
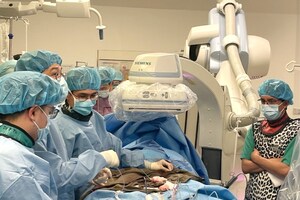
LONDON and HANGZHOU, China, Sept. 19, 2016 /PRNewswire/ -- Venus P, the transcatheter pulmonary valve developed in-house by Hangzhou, China-based medical instrument producer Venus Medtech (Hangzhou) Inc., was successfully implanted in a European patient in London on September 13th, 2016. Venus Medtech is the sole owner of the intellectual property rights to the valve. This is not only a milestone for the official launch of CE Mark certification clinical trials being undertaken for Venus Medtech's Venus P-Valve, but also marks the start of a journey for the entry of China-made transcatheter valve in the developed markets of the U.S. and Europe.
The first patient who participated in the trial received a successful implant of the Venus P-Valve, with the operation performed by Prof. Shakeel Qureshi at Evelina Children's Hospital in London on September 13th, 2016. The 27 year-old female patient suffered significant pulmonary regurgitation following surgery for tetralogy of Fallot. And she didn't show any signs of the regurgitation after the successful implant and the valve works well. The patient's vital signs including blood pressure have remained within normal ranges since the operation. The doctors were quite impressed with the clinical performance of the Venus P-Valve. Venus P-Valve is designed to treat pulmonary regurgitation with or without stenosis in patients.
Prof. Shakeel Qureshi is the Principal Investigator (PI) for the CE Mark certification clinical trial in Europe, the first phase of the clinical trials of which involved 6 locations, and 80 to 100 patients across Europe, including a few locations outside of the continent. This is the first formal set of clinical trials in multiple locations across Europe that used China-made transcatheter valves.
Venus Medtech is fully dedicated to the research and development of medical instruments for cardiac valves that require minimally invasive treatment. The firm has twice been the first worldwide to produce the models for new valve systems: the pre-loading transcatheter valve system and the transcatheter self-expanding pulmonary valve system.
Venus Medtech (Hangzhou) Inc., located in Hangzhou National Hi-tech Development Zone (Binjiang District), focuses their R&D on internationally advanced artificial valve systems with commercial applications and has gone a long way in satisfying the need for such products both in China and around the world.
For more information, please visit http://en.venusmedtech.com/



Share this article